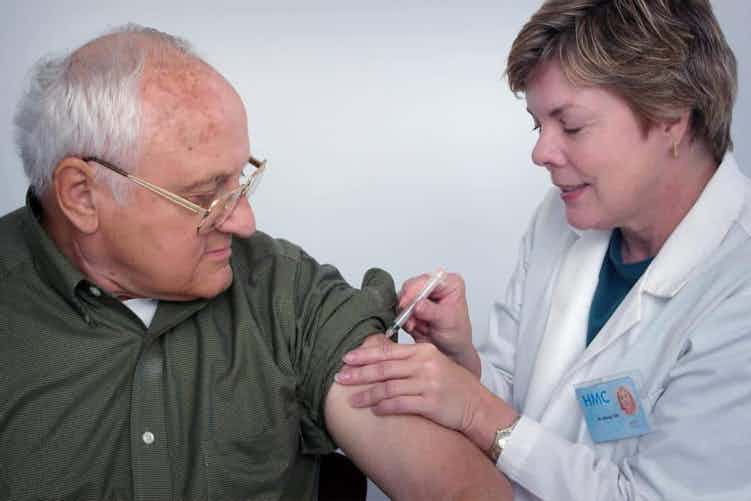
Adults in their 60s and 70 have responded well in trials to a COVID-19 vaccine developed in Oxford.
Researchers working on the vaccine tested 560 healthy volunteer adults. They concluded that the findings were “encouraging”.
This is the second phase of the vaccine trial, which was testing to see whether people developed side effects from having the jab. Study lead from the University of Oxford, Professor Andrew Pollard, told the BBC that he was "absolutely delighted with the results" as even participants in their 70s showed a good immune response.
Dr Maheshi Ramasamy, who was one of the investigators within the Oxford vaccine group, is also pleased after 99% of the participants demonstrated neutralising antibodies.
The official results from phase two testing are expected in the weeks to come.
Currently, the Pfizer-BioNTech, Sputnik, and Moderna vaccines have already published data from their phase three trials, which tested whether the vaccine would prevent people from getting COVID-19. Pfizer-BioNTech reported that 94% of adults over 65 could be protected.
The Oxford vaccine will be carrying out their phase three trials in the weeks to come. Pollard said he was hoping to have the data of phase three released “before Christmas”.
AstraZeneca will manufacture the Oxford vaccine. The UK government has confirmed they have ordered 100 million jabs of the Oxford vaccine and 40 million of the Pfizer-BioNTech vaccine. In addition, 5 million Moderna vaccines are also on order.
Talking on the rate of competition among the different developers of the coronavirus vaccine, Pollard said there was "no competition" and that “we will need all of them to protect people around the globe."
Many now see these developments as a step in the right direction to a normal Christmas. However, experts are saying that there should be a planned relaxing of the lockdown.
Professor Andrew Hayward of the UCL Institute of Epidemiology and Health Care said gatherings among families and loved ones at Christmas time would pose "substantial risks" to increasing infection rates.
Speaking to BBC Radio 4, Hayward said, "We're on the cusp of being able to protect those elderly people, who we love, through vaccination.
"It would be tragic to throw that opportunity away... by trying to return to normality over the holidays.
"My personal view is we're putting far too much emphasis on having a near-normal Christmas," he added.